ISO 14971 provides a framework for medical device manufacturers to identify and minimize risks through the design and manufacturing processes. Adhering to the standard helps ensure overall safety and efficacy.
At its core, Risk Management According to ISO 14971 involves analyzing potential hazards and estimates of severity. Manufacturers must determine appropriate risk control procedures and evaluate residual risk. Matrix software allows teams to streamline and document these critical activities. Its comprehensive features provide solutions at each step for meeting quality requirements.
With Matrix, users gain visible traceability over the life cycle risk assessment process specified by ISO 14971. Whether designing, developing or improving products, the standard and software work hand-in-hand to address risks proactively and enhance patient outcomes.
What is Risk Management?
Risk management is the process companies use to identify and understand potential problems before they happen.
By examining what could go wrong and how bad it might be, risk management helps prioritize the most significant risks. It then focuses on reducing risks by changing plans or adding safeguards. The goal is to protect the business or project from threats that could cause trouble.
Regular reviews spot new risks, too. This method helps organizations operate smoothly with fewer risky surprises.
What Are the Benefits of Iso 14971?
Here are some benefits you need to know:
- Patient Safety
ISO 14971 requires identifying hazards and estimating risks to patient health. This ensures medical devices address safety concerns proactively. Following the standard leads to better products that benefit patients without causing harm.
- Regulatory Compliance
Adhering to ISO 14971 helps medical devices comply with global regulations like the FDA and EU. This facilitates market access and saves time and money by streamlining the regulatory approval process.
- Continuous Improvement
The standard promotes continually reviewing risk processes throughout a device’s lifecycle. This helps organizations learn from experience and prioritize improvements to reduce patient health and safety risks.
- Defensibility of Design Process
ISO 14971-compliant risk files create clear records ideal for regulatory audits and investigations. They demonstrate that a robust, well-designed approach was followed if questions arose regarding product safety.
- Consistent Approach
The standard provides a uniform framework regardless of device or company. This consistency benefits stakeholders by outlining standard, industry-recognized best practices for risk processes.
- Peace of Mind
Following ISO 14971 reassures management that risk-based thinking influences product development decisions appropriately. This peace of mind allows a more significant focus on core operations and serving customers.
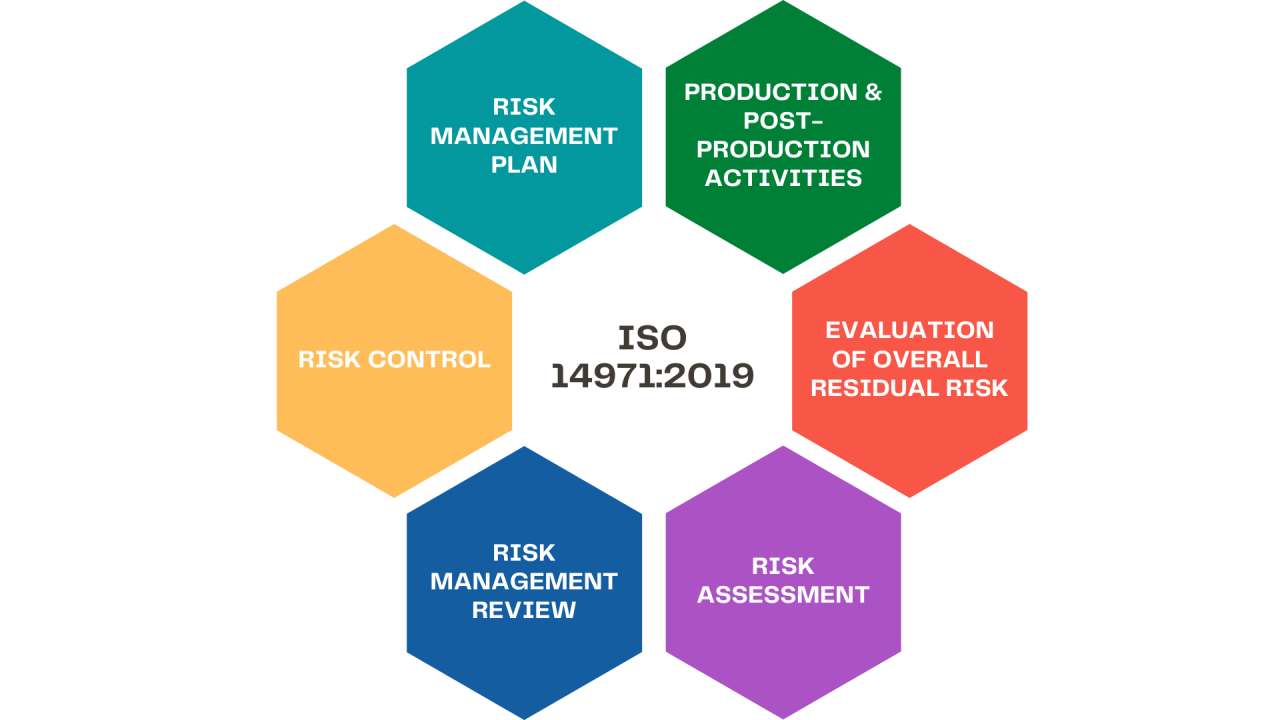
The ISO 14971 Risk Management Process
Here are the key steps of the ISO 14971 risk management process:
- Risk Management Plan
The plan establishes the detailed scope, objectives, roles and responsibilities for identifying, assessing, controlling and monitoring risks associated with the device throughout its lifecycle.
- Risk Analysis
A comprehensive analysis is conducted to identify all known and foreseeable hazards and estimate the severity and likelihood of potential harm from each hazard, which helps prioritize the risks.
- Risk Evaluation
Each risk identified during analysis is systematically evaluated based on specified criteria to determine if the level of risk related to each hazard is acceptable or requires further risk reduction measures.
- Risk Control
Appropriate control measures are selected and applied to eliminate hazards or sufficiently reduce unacceptable risks during evaluation to achieve the best balance of benefit and risk.
- Residual Risk Evaluation
Any remaining risks after implementing controls are reassessed to confirm they are as low as reasonably practical and acceptable according to criteria before releasing the product.
- Risk Management Report
A detailed report documents the entire risk management process, methods, and findings to provide full traceability and demonstrate compliance with regulators’ standards.
- Post-Production Monitoring
Data on device performance and any adverse events during use are monitored and analyzed continuously to ensure risk control adequacy and identify needs for continual improvement.
Support Risk Management with QMS
Quality management systems (QMS) are essential in risk management activities.
A QMS establishes procedures for processes like design and development, manufacturing, corrective and preventive action and document control. This provides a robust system for implementing risk management plans, tracking risk assessments, and ensuring adequate controls.
Key risk management documents like the risk file can also be maintained as controlled QMS records. An integrated approach optimizes the benefits of both QMS and risk management standards.
Conclusion
ISO 14971 provides a framework for identifying and mitigating risks throughout a medical device’s lifecycle. Matrix software streamlines this crucial process.
Users can assess hazards, design controls, and monitor residual risks in a centralized system with full traceability. This gives insight to make informed decisions. Ultimately, following ISO 14971 guidelines and leveraging dedicated risk management software like Matrix helps medical technology developers balance innovation and patient safety.
Companies implementing robust risk controls early on through standards and solutions will be best positioned to navigate industry challenges. Most importantly, they can achieve their shared goal of helping practitioners heal.