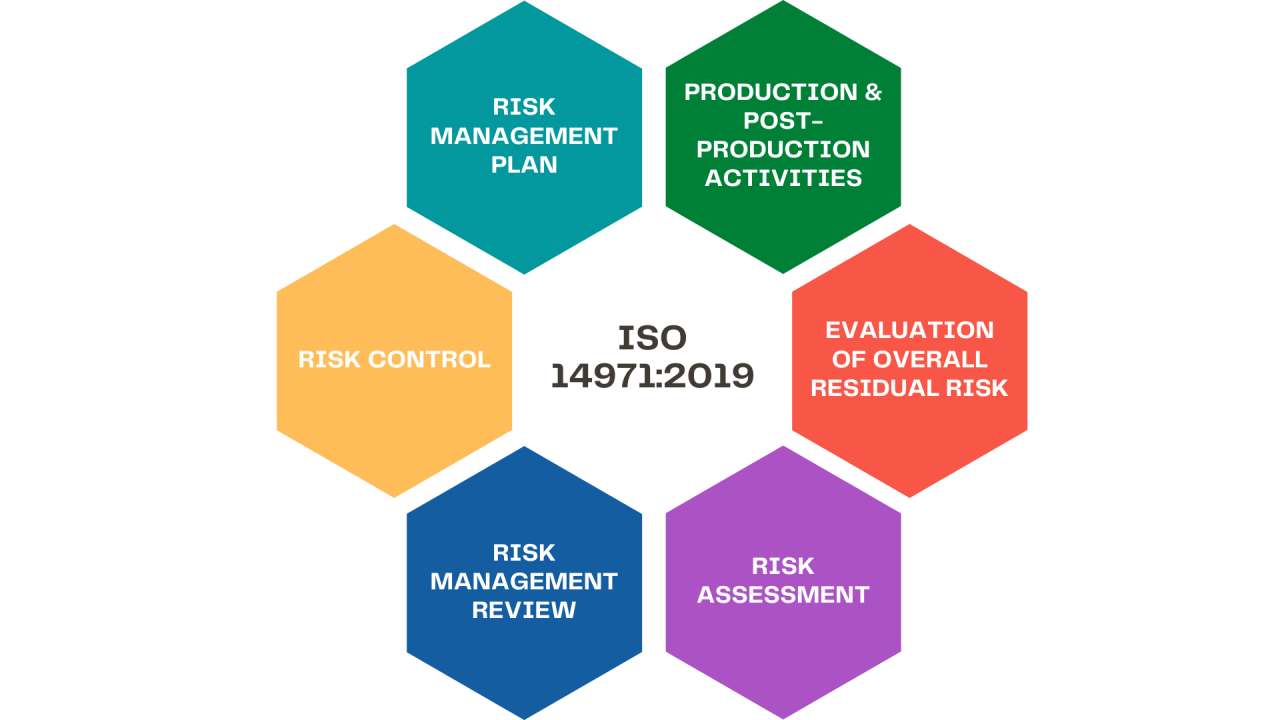
What is Risk Management According to ISO 14971 for Medical Devices?
ISO 14971 provides a framework for medical device manufacturers to identify and minimize risks through the design and manufacturing processes. Adhering to the standard helps ensure overall safety and efficacy. At its core, Risk Management According to ISO 14971 involves analyzing potential hazards and estimates of severity. Manufacturers must determine appropriate risk control procedures and … Read more